“Describe the process of drug discovery from nature. Choose a plant that has been used as the source material for a conventional medicine as an example.”

introduction
This essay describes the process of the discovery of digoxin, a cardiac glycoside derived from Digitalis spp..
To begin, this essay will observe the plant Digitalis purpurea L. (IPNI) and then continue by looking at traditional uses of the plant and its formal entrance in to medicine. The importance of standardisation and preparation will be considered, as will isolation of active constituents and their actions.
In particular, the cardenolides, Digoxin and Digitoxin will be examined, as well as the administration, contra-indications and toxicity of the drug.
The essay will end by comparing the preclinical studies and clinical trials of D. purpurea with what would be expected in modern times and look at recent systematic reviews of digoxin.
Illustration 1: Digitalis purpurea (Kohler 1887)
Botanical description:
D. purpurea, otherwise known as foxglove, common foxglove and purple foxglove (Chevallier 2016, POWO), is a tall (up to 150cm), biennial plant common in Europe and North America. (Dewick 1997). It has pubescent stems, leaves and flower stalks and pinkish-purple tubular flowers from June to September. Foxglove favours woodland clearings on acid, well drained soil but can also be found on hedge banks, open clearings and rocky hillsides. (Streeter et al 2016)
Traditional use and discovery
In Anglo-Saxon times the plant was seen as unlucky and connected to super-natural entities but was used as an application to the breasts to cease lactation. (Pollington 2000)
The leaves (EB 2004) of D. purpurea were traditionally used in Medieval Europe to treat a number of ailments including epilepsy, headaches and spasms, TB and dropsy (Dauncey and Larsson 2018, Griggs 1997).
Pharmacologist and botanist, W Withering (1741-1799), was responsible for establishing the use of D. purpurea as a treatment for dropsy (oedema). He was inspired by an ‘old woman in Shropshire’ who used a renowned family recipe containing Foxglove. (Dauncey and Larsson 2018, Griggs 1997).
Withering fed Foxglove leaves to turkeys, observing that standardisation and dosage were of great importance to avoid toxicity. Withering examined administration of D. purpurea in 163 cases over 10 years (Dauncey and Larsson 2018, Griggs 1997) and published a summery of his observations in ‘An Account of the Foxglove, and Some of Its Medical Uses‘ (1785). (EB 2004).
Following Withering’s publication, the use of D. purpurea gradually became accepted as a treatment for dropsy, (Dauncey and Larsson 2018) irregular heart rhythms and congestive heart failure (WebMD).
Standardisation, preparation and Isolation
For potent activity first year leaves are harvested and quickly dried at 60 degrees Celsius. Improper drying affects the cardiac glycosides due to hydrolysation, resulting in less active constituents. (Dewick 1997).
In order to create a standardised leaf powder, the herb was assessed on the heart muscles of guinea pigs and pigeons, allowing stronger and weaker batches to be combined to create a standardised strength. (Dewick 1997)
The entire leaf is now rarely used; pharmaceuticals contain pure, isolated glycosides. (Dewick 1997)
Digoxin, the most commonly used isolated compound of Digitalis spp., was first isolated in Britain in 1930 by Dr Sydney Smith, from the leaf of D. lanata (Woolly foxglove). (Hollman,1996)
Active constituents and their actions
(Chevallier 2016, Dewick 1997)
| Table 1: Active constituents of Digitalis purpurea (fol) |
| cardiac glycosides (0.15-0.4%) (cardenolides): digoxin; digitoxin; lanatosides |
| anthroquinones |
| flavonoids |
| steroidal saponins: tigogenin; neotigogenin |
D. purpurea s most known for it’s cardiac glycosides but contains multiple constituents with potential medical benefits, As shown in Table 1.
Cardiac glycosides are a secondary metabolite, of triterpenoid origin, biosynthesised by the Mevalonate pathway, from the metabolism of cholesterol. Cardiac glycosides inhibit Na+, K+, ATPase in the cell walls of the heart muscle, leading to an increase in intercellular Ca2+ concentration and stronger contractions. (Dewick 1997, Hoffman 2003)
The pharmacological effects of cardiac glycosides are made possible by the attached aclycones, which are steroidal in nature (Henreich et al 2015), and modified by the sugar at C3, which allows the glycosides to be soluble enough to bind to the heart muscle. (Hoffman 2003).
However as the solubility and removal rates of cardiac glycosides tend to be low, there is a possibility of accumulation with in the body, which would lead to toxicity. (Hoffman 2003). The therapeutic dose 50-60% of the toxic dose. (Dewick 1997)
Used therapeutically, the cardiac glycosides in D. purpurea increase myocardial contraction and reduce conductivity within the atrioventricular node (NICE). Indicated for increasing the heart output (EB 2004) and improving irregular heart rhythms (WebMD)
Cardenolides: Digitoxin and Digoxin
Cardenolides are C23 cardiac glycoside structures, with an unsaturated five membered lactone ring at C17. They are found in found in Digitalis spp. and some other plants.
Cardenolides are cardio-active, possess an alkene, and a cyclic ester. (Heinreich et al 2015, Dewick 1997)
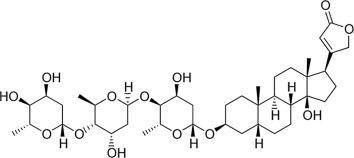
Illustration 2: Digitoxin (Researchgate)
Digitoxin and digoxin are the cardenolides isolated for pharmaceuticals. (Chevallier 2016) Their effect on the vagus nerve means they can be used to control atrial arrhythmias. (Hoffman 2003)
Digitoxin contains 5 OH groups (Researchgate). It is rapidly strengthens the heartbeat but as it is excreted from the body slowly as it is metabolised by the liver. digoxin is preferred as a long term medication (Chevallier 2016, Dewick 1997)
Digoxin contains 6 OH groups (Pubchem). Digoxin inhibits the sodium potassium adenosine triphosphatase (ATPase) pump, enhancing cardiac contractility (Hoffman 2003, Pubchem) and suppresses conduction on the atrioventricular node. It is used to control ventricular rate in atrial fibrillation and in the management of congestive heart failure with atrial fibrillation
(Pubchem).
Digoxin is more hydrophillic than digitoxin and binds less strongly to plasma proteins. It is eliminated by the kidneys and is therefore preferred as a long term medication as there is less risk of accumulation. (Dewick 1997)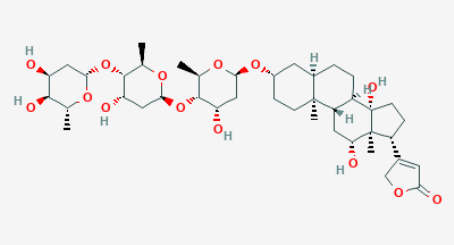
Image 3: Digoxin (Pubchem)
Dosage
Digoxin can be taken in tablet, liquid or injection form. In the UK, the brandname for digoxin is ‘Lanoxin’. Adult dosage is shown in Table 2.
The onset of action when taken by mouth is 1-2 hours and duration is up to 4 days. Possible adverse effects are usually due to increased levels of the drug in the blood and periodic checks on blood levels of Digoxin may be advisable. Digoxin may be more toxic in those with depleted potassium levels (BMA 2001)
Table 2: Digoxin dosage advice (NICE)
| Atrial fibrillation or flutter | heart failure (patients in sinus rhythm) | |
| Adult dose for rapid digitalisation | 0.75–1.5 mg in divided doses, dose to be given over 24 hours, reduce dose in the elderly. by mouth | |
| Adult dose for maintainance | Maintenance 125–250 micrograms daily, dose according to renal function and initial loading dose, reduce dose in the elderly.by mouth | |
| Adult | 62.5–125 micrograms once daily, reduce dose in the elderly.
by mouth |
Contra indications
Digitalis is contra-indicated for those on any medication which affects the heart (WebMD).
Long-term use of foxglove can lead to symptoms of toxicity, including visual halos, yellow-green vision, and stomach upset. (Dauncey and Larsson 2018)
Foxglove can cause irregular heart function and death. Signs of foxglove poisoning include stomach upset, small eye pupils, blurred vision, strong slow pulse, nausea, vomiting, dizziness, excessive urination, fatigue, muscle weakness and tremors, stupor, confusion, convulsions, abnormal heartbeats, and death. (Dauncey and Larsson 2018)
Although it is generally advised to avoid Digoxin during pregnancy and breastfeeding (WebMD), the BMA reports there is no evidence of risk during pregnancy, although the dose may be adjusted and that, although Digoxin passes into the breastmilk adverse effects on the baby are unlikely at normal dosage. (BMA 2001)
Digoxin is contra-indicated for those with kidney disease, or in those who are taking medication which effects the passing of waste from the body (ie, some antibiotics, diuretics and laxatives), due to the increased risk of Digoxin accumulation. (WebMD).
BMA state the overdose danger rating of Digoxin as high. Adverse effects which may indicate the toxic dosage has been reached include severe tiredness, nausea, confusion, visual disturbances, palpitations.(BMA 2001)
There are also many medications (including some herbs) which may reduce levels of digoxin in the blood (EMC 2018). There is a digoxin specific antibody available for reversing life-threatening overdose, as well as biological assays. (Dewick 1997)
Preclinical studies and clinical trials
Withering carried out studies and trials in a way that is recognisable today, observing case studies, incorporating animal experimentation and then enlarging and observing the population of patients
Drugs destined for the human market are subject to numerous tests to ensure their safety. Prior to clinical trials on patients, a number of preclinical studies take place which may include in vitro studies of cell, tissue and organ cultures, computerised models and in vivo experimentation using animals to test safety and efficacy. (Torjeses 2015).
Clinical trials are carried out in phases – gradually increasing numbers of patients observed. Following licensing clinical trials may continue for safety surveillance, or ‘pharmacovigilance’ (Torjeses 2015).
Research continues on digoxin: Two systemic reviews on the use of digoxin were published in 2015. One concluded that, due to the potential risks and benefits, and the presence of alternative drugs, the role of digoxin in the management of patients with normal sinus rhythm and congestive heart failure is limited and there may be a risk of increased mortality in those with atrial fibrillation alone. However, in patients with both atrial fibrillation and systolic congestive heart failure, or following MI, further studies should be done. (Virgadamo et el 2015).
The second concluded that digoxin has a neutral effect on mortality and a lower rate of admission to hospital across all study types. (Ziff et al 2015)
Conclusion
This essay has described the process of drug discovery from nature using D. purpurea as an example. Observing the plant, it’s traditional uses and formalisation into medicine has demonstrated early research. The essay moved on to observe the isolation and uses of the cardenolides. The molecular structure of digoxin and digitoxin were examined. Modern uses of digoxin have been discussed and the systematic reviews mentioned have demonstrated how the plant based medicine digoxin is standing up to modern scrutiny.
Word count: 1573.
References:
- BMA (British Medical Association) (2001) New Guide to Medicines and Drugs 5th ed. London: Dorling Kindersley Limited
- Chevallier A (2016) Encyclopedia of Herbal Medicine , 3rd ed. London: Dorling Kindersley Ltd
- Dauncey A, Larsson S (2018) Plants That Kill New Jersey: Princeton University Press
- Dewick P (1997) Medicinal Natural Products West Sussex:John Wiley and Sons Ltd
- EB (Encyclopaedia Britannica) (2004) Digitalis Available: https://www.britannica.com/science/digitalis [accessed 18/12/18]
- EMC (2018) Lanoxin Tablets 0.25mg Available:https://www.medicines.org.uk/emc/product/5465/pil [accessed 21/12/18]
- Griggs B (1997) Green Pharmacy Vermont: Healing arts Press
- Heinreich M, Barnes J, Gibbons S, Williamson E A (2015) Fundamentals of Pharmacognosy and Phytotherapy, 2nd ed. Edinburgh: Churchill Livingstone Elsevier
- Hoffman D (2003) Medical Herbalism Vermont:Healing arts Press
- Hollman A (1996) Digoxin comes from Digitalis lanata Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2350584/?page=1 [accessed 20/12/18]
- IPNI (International Plant Names Index) Digitalis purpurea Available: http://www.ipni.org/ipni/simplePlantNameSearch.do;jsessionid=3B824CE936794DCBEF3539D8D6A72884?find_wholeName=Digitalis+purpurea&output_format=normal&query_type=by_query&back_page=query_ipni.html [accessed 19/12/18]
- Kohler (1887) Digitalis purpurea [image – coloured plate] Available http://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:802077-1
- NICE (National Institute of Clinical Excellence) Digoxin Available: https://bnf.nice.org.uk/drug/digoxin.html [accessed 15/12/18]
- Pollington S (2000) Leechcraft Early Charms Plantlore and Healing Cambridgeshire: Anglo-Saxon Books
- POWO (Plants of the World Online) Digitalis purpurea L.Available: http://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:802077-1 [accessed 19/12/18]
- Pubchem Digoxin Available: https://pubchem.ncbi.nlm.nih.gov/compound/digoxin#section=2D-Structure [accessed 19/12/18]
- Researchgate Chemical structure of digitoxin Available: https://www.researchgate.net/figure/Chemical-structure-of-digitoxin-C41H64O13-the-molecule-contains-five-OH-groups_fig3_306387276 [accessed 19/12/18]
- Streeter D, Hart-Davies C, Hardcastle A, Cole F, Harper L. (2016) Wild Flower Guide 2nd ed. London:William Collins
- Torjeses I (2015) Drug development: the journey of a medicine from lab to shelf Available: https://www.pharmaceutical-journal.com/publications/tomorrows-pharmacist/drug-development-the-journey-of-a-medicine-from-lab-to-shelf/20068196.article?firstPass=false [accessed 21/12/18]
- Virgadamo S, Charnigo R, Darrat Y, Morales G, Elayi C (2015) Digoxin: A sysematic review in atrial fibrilation, congestive heart failure and post myocardial infarction Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4660476/ [accessed 21/12/18]
- WebMD Foxglove Available: https://www.webmd.com/vitamins/ai/ingredientmono-287/foxglove [accessed 15/12/18]
- Ziff O, Lane D, Samra M, Griffith M, Kirchhof P, Lip G, Steeds R, Kotecha D (2015) Safety and efficacy of digoxin: systematic review and meta-analysis of observational and controlled trial data Available: https://www.bmj.com/content/351/bmj.h4937 [accessed 21/12/18]

 Process Of Drug Discovery From Nature
Process Of Drug Discovery From Nature